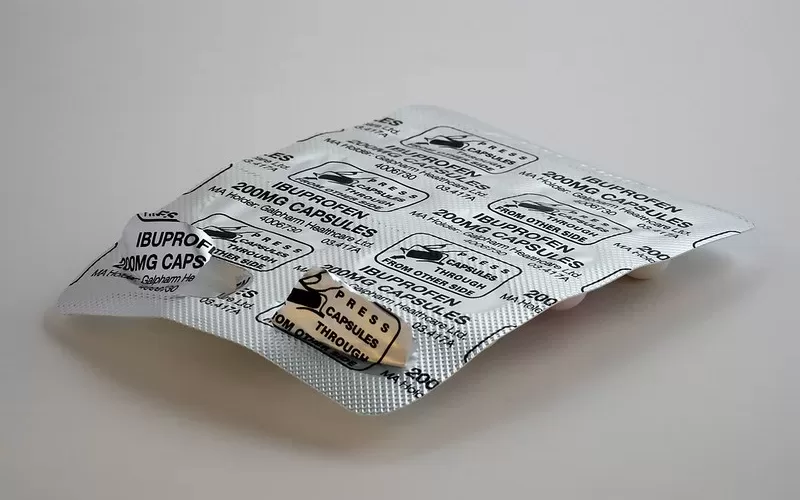
In the highly regulated pharmaceutical industry, exceeding stringent standards is critical. It’s not just an advantage, it’s a requirement for product safety. It is essential for maintaining consumer confidence. Flexo printing has consolidated its position in this important area by helping blister packs achieve compliance. It is particularly important in the production of blister packs. It is also important in the application of adhesives. These processes must meet strict regulatory requirements.
Flexo Printing in Blister Pack Production
Multi-Material Adaptability
The ability of flexo printing to work harmoniously with a wide range of materials, including various plastics and films, is fundamental to ensuring compliance. This versatility ensures that pharmaceutical companies can produce blister packs that are not only durable, but also maintain the purity and safety essential for direct drug contact. It’s this adaptability that preserves the integrity of critical product details and brand identity.
High-Quality Output
Flexo’s superior resolution means that every blister pack has crisp, legible text and images. This level of clarity is critical for clearly communicating product details, dosing guidelines and expiration dates to consumers and healthcare providers alike, simplifying product identification and enhancing patient safety.
Adhesive Compliance in Pharmaceutical Packaging
Improved Accuracy and Compatibility
Flexo’s advancement in adhesive application precision ensures consistent quality and bond strength throughout the package. This accuracy is key to creating blister packs that are both secure and tamper evident, meeting the stringent standards for package integrity and security.
Adhesives Regulatory Compliance
Application flexibility enables the use of adhesives that comply with the extensive health and safety regulations imposed by authoritative bodies such as the FDA and EMA. By using compliant adhesives, pharmaceutical manufacturers can ensure that their blister packs maintain drug efficacy and patient well-being in accordance with demanding global standards.
How Flexo Printing Helps Blister Packs Achieve Compliance
Traceability Features
Efficient embedding of traceability elements, such as barcodes and serial numbers with flexo printing, is essential for compliance with regulations requiring comprehensive track and trace systems. This functionality facilitates effective tracking of the product lifecycle from production to the end consumer, ensuring product authenticity and safety.
Quality and Safety Standards
Adherence to Quality and Safety Protocols for flexo printing in blister and adhesive production ensures that the results meet the stringent requirements of regulatory bodies. Such compliance mitigates the risks associated with product recalls, regulatory sanctions, and reputation damage.
Case Study
The real-world effectiveness of flexo printing can be seen in specific industry examples. A well-known pharmaceutical company converted its blister pack production to flexo, not only meeting FDA requirements but also achieving a remarkable improvement in packaging quality. Similarly, a European pharmaceutical company leveraged the precision of flexo for adhesive application, significantly reducing the risk of contamination and exceeding EMA safety expectations.
In Summary
Integrating flexo printing into pharmaceutical packaging is a sophisticated strategy. It effectively navigates the complexities of regulatory compliance. Flexography’s adaptability is unmatched. Its precision is unsurpassed. It can seamlessly incorporate traceability features. This puts flexography at the forefront of packaging innovation.
Flexo printing ensures that blister packs and adhesives are of the highest quality. It strictly adheres to compliance standards. This is critical to patient safety. It also preserves the integrity of pharmaceutical products. As regulatory frameworks evolve and become more complex, flexography’s contribution to the industry is both undeniable and indispensable.